The Next-Generation Longevity Formula Designed To Slow Down Aging At A Cellular Level


What You Can Expect From Longenesis
- Noticeably increased energy levels
- Improved immune function
- Improved mitochondrial function
- Improved focus, clarity, and brain energy levels
- Reduced inflammation (chronic low-grade inflammation is a major driver of aging and disease)
- Long-term decreased risk of neurodegenerative diseases, cardiovascular diseases, and numerous other diseases
- Dramatically improved resilience, faster recovery, and resistance to the effects of stress
- Powerful ingredients backed by science showing actual reductions in rates of disease, longer lifespans, and more youthful measurements of validated biological markers of aging.
Aging Is A Complex Process
Aging is a complex process that is now understood by gerontologists (aging scientists) to be driven by at least 10 key mechanisms. These include:
- Mitochondrial dysfunction
- Inflammation (“Inflammaging”) and disrupted cell communication
- Cellular senesence (“zombie” cells that secrete pro-inflammatory molecules that damage neighboring cells)
- Protein accumulation inside and outside of cells that hinders cell functions
- Epigenetic dysregulation (dysfunctional signaling leading to non-optimal changes in which changes are switched on or off)
- Oxidative damage
- Dysfunctional DNA methylation
- Suppressed autophagy and mitophagy (the quality control process of maintaining healthy cells and mitochondria)
- Reduced stem cell proliferation
- Telomere shortening
- (And other mechanisms, like DNA damage, glycation of tissues, and more).
"When designing Longenesis, our goal was to create the world’s most comprehensive formula using science-backed ingredients proven to impact on these mechanisms of aging."
For example, there are ingredients in the formula like ca-AKG proven to act on several of these mechanisms like improving mitochondrial health, reducing inflammation, reducing oxidative damage, and altering epigenetic signaling (gene expression). Or spermidine, which powerfully stimulates autophagy to allow the body to recycle and repair damaged cell parts. Or fisetin, which has been shown to powerfully clear away senescent cells. Or glucosamine sulfate, which has been shown to transiently challenge mitochondria (like exercise for mitochondria), making them larger and increasing their energy producing capacity – and which has been shown to actually translate into the hard outcomes of increased lifespans in animal models, and has been strongly associated with longer lifespans in humans.
All of this is just scratching the surface of the powerful ingredients in Longenesis, and the dozens of mechanisms through which these ingredients are provent to slow down the rate of cellular aging. (A more in-depth examination of the ingredients and the science behind them is below).
In addition, there is an enormous focus on safety in our formula. While there are certain ingredients that may enhance longevity, their side effect profile is highly questionable. Things like rapamycin, C60, and metformin (and many other new synethic compounds) fall into this category. In Longenesis, we’ve included only ingredients which have extremely good safety profiles and virtually no risk of harm – and which are natural (rather than synthetic lab-made molecules).
You Can Slow Down The Process Of Cellular Aging!
To do this you must:
- Reduce the oxidative stress and inflammation that drives mitochondrial damage and dysfunction
- Improve your stem cell production and proliferation
- Clear senescent cells more effectively
- Build up your internal antioxidant and detoxification defense systems
- Optimize your genetic expression through proper epigenetic signaling
- Reduce or eliminate chronic low-grade inflammation
- Protect your telomeres
- Restore proper DNA methylation
- Rebuild your mitochondria
This is what Longenesis has been designed to do.
Buy Longenesis Now
1 Month Supply

Longenesis
$119
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Subscribe & Save


Longenesis
$119.00 $89.00
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
3 Month Supply

Longenesis
$299
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Buy Longenesis Now
1 Month Supply

Longenesis
$119 $89.00
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Subscribe & Save


Longenesis
$119.00 $77.35
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
3 Month Supply

Longenesis
$357 $267.00
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Buy Longenesis Now
1 Month Supply

Longenesis
$119
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Subscribe & Save


Longenesis
$119.00 $89.00
SAVE $30 Per ITEM
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
3 Month Supply

Longenesis
$299
SAVE $19 Per ITEM
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
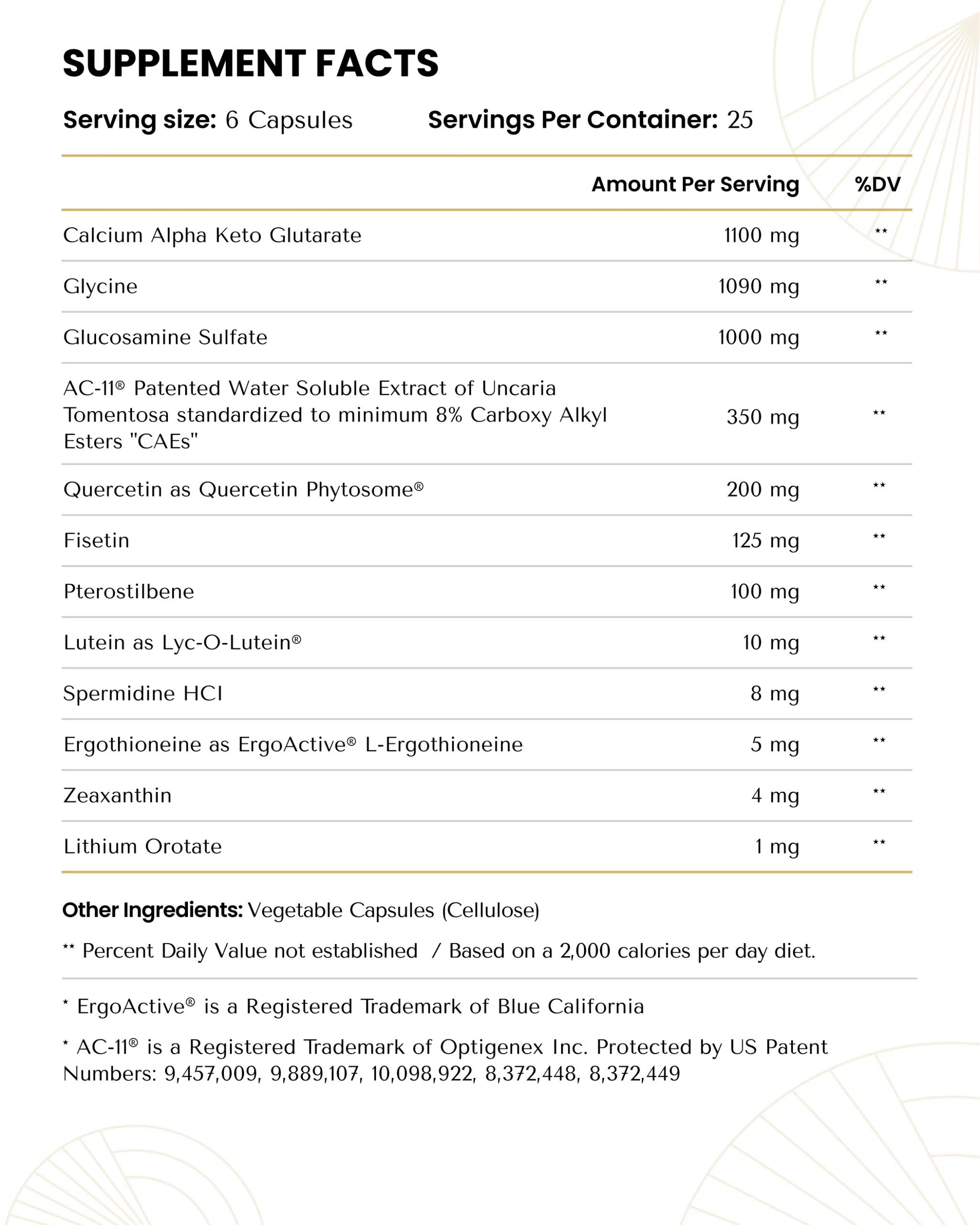
The Perfect Formulation
12 premium, carefully sourced, and science-backed ingredients in their optimal dosages

Alpha-ketoglutarate
Alpha-ketoglutarate (AKG) is a key molecule in mitochondrial energy production that determines the overall rate of electron generation within the tricarboxylic acid (TCA) cycle. It is also essential for the function of several enzymes involved in gene expression and collagen synthesis. These unique roles have implicated AKG in healthy aging longevity [1].

Glycine
Glycine is a conditionally essential amino acid that wears many hats. It is necessary for protein synthesis, particularly that of collagen (the most abundant protein in the body), and an essential component of several biologically important compounds like porphyrins and heme, creatine, glutathione, and purines. It is also an important signaling molecule within the nervous and immune systems.

Glucosamine Sulfate
Glucosamine is a naturally occurring component of crustacean exoskeletons and fungi. Best known for reducing joint pain and improving mobility, few know of its profound mitochondrial benefits and lifespan-extending effects [25].

AC-11
AC-11 is a patented extract of Uncaria tomentosa, also known as cat’s claw, that enhances the body’s natural DNA repair mechanisms. DNA damage is a central feature of the aging process, and its reduction can help counteract virtually all age-related diseases [33].

Quercetin Phytosome
Quercetin is a flavonoid with anti-inflammatory, antioxidant, and antiviral properties [41]. In particular, quercetin is able to inhibit the ability of respiratory viruses to enter cells and replicate, essentially reducing the risk of infection [42,43]. It also acts as a zinc ionophore, meaning that it carries zinc ions into cells so that the zinc can prevent viral replication [44].

FISETIN
Fisetin is a naturally occurring flavonol found in fruits and vegetables best-known for its potent senolytic effects — it’s ability to kill off senescent cells that contribute to aging, disease, and dysfunction throughout the body.

Pterostilbene
Pterostilbene is a natural molecule found in fruits, vegetables, and nuts, the best source being blueberries, and is a structural cousin to resveratrol. In supplemental doses, it confers a variety of health benefits that delay the aging process.

Lithium
Lithium is a non-essential mineral found primarily in rocks. While best-known for its psychotropic effects and use as a psychiatric medication among those with bipolar disorder, schizophrenia, and depression, it can have a variety of anti-aging benefits when taken at much lower “microdoses”.

Lutein & Zeaxanthin
Lutein and zeaxanthin are carotenoids abundant in green leafy vegetables and egg yolk that preferentially accumulate in the retina and its supporting structures to protect the eyes against oxidative stress from light [61], which has massive implications for age-related vision loss. Lutein and zeaxanthin protect the eyes in two ways [62–64]: They situate themselves in front of delicate functional structures of the retina, such as photoreceptors, and directly absorb blue light (about 40%, on average) before it ever reaches the structures and has a chance to cause oxidative stress.

Spermidine
Spermidine is a naturally occurring polyamine that we synthesize and obtain from food. It regulates a variety of biological processes such as cell growth and proliferation, tissue regeneration, DNA and RNA stabilization, enzymatic modulation, and regulation of translation, among others.

Ergothioneine
Ergothioneine is a naturally occurring amino acid that humans cannot synthesize and instead obtain exclusively through their diet. After consumption, it accumulates throughout the body, where it plays a role in many age-related diseases [94]. While levels increase through young adulthood, they begin to decline in old age [95–97].
Complete Transparency on the Label
As always, we operate with complete transparency with the ingredients in our formulas. No “proprietary blends” to hide miniscule dosages of ingredients (as most manufacturers do). We proudly list out all our ingredients and their actual dosages, with nothing to hide.
While most manufacturers list out an impressive list of ingredients, what they don’t tell you is that they are typically using 1/5th, 1/10th, or even 1/20th of the proper clinically effective dosages of those ingredients that were actually used in the studies and shown to have benefits. As always, the dose makes the medicine! Just as there is a big difference in workouts between walking 20 steps vs. running a marathon, there is a big difference between using the proper 1,000 mg dose of a compound vs. using only 50 mg. These do not and will not have remotely the same benefit.
We encourage you to compare the ingredients, and the dosages of each ingredient to any other longevity formula on the market. You’ll quickly see that our formula is radically more comprehensive in terms of ingredients, and simultaneously uses much larger doses – the clinically effective dosages that are actually used in studies and proven to have benefits.
Buy Longenesis Now
1 Month Supply

Longenesis
$119
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Subscribe & Save


Longenesis
$119.00 $89.00
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
3 Month Supply

Longenesis
$299
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Buy Longenesis Now
1 Month Supply

Longenesis
$119 $89.00
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Subscribe & Save


Longenesis
$119.00 $77.35
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
3 Month Supply

Longenesis
$357 $267.00
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Buy Longenesis Now
1 Month Supply

Longenesis
$119
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Subscribe & Save


Longenesis
$119.00 $89.00
SAVE $30 Per ITEM
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
3 Month Supply

Longenesis
$299
SAVE $19 Per ITEM
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
Worldwide Shipping (FREE Shipping within the U.S.)*
* For international orders: All shipments are shipped Duties, Customs, & Taxes unpaid. Buyer is responsible for delivery fees.
References
All References
- Bayliak MM, Lushchak VI. Pleiotropic effects of alpha-ketoglutarate as a potential anti-ageing agent. Ageing Res Rev. 2021;66:101237.
- Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol Ther . 2016;24:1–8.
- Harrison AP, Pierzynowski SG. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art–review article. J Physiol Pharmacol. 2008;59 Suppl 1:91–106.
- Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602.
- Karemaker ID, Vermeulen M. ZBTB2 reads unmethylated CpG island promoters and regulates embryonic stem cell differentiation. EMBO Rep [Internet]. 2018;19. Available from: http://dx.doi.org/10.15252/embr.201744993
- Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016;24:542–54.
- Asadi Shahmirzadi A, Edgar D, Liao C-Y, Hsu Y-M, Lucanic M, Asadi Shahmirzadi A, et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020;32:447–56.e6.
- Wang Y, Deng P, Liu Y, Wu Y, Chen Y, Guo Y, et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat Commun. 2020;11:5596.
- Demidenko O, Barardo D, Budovskii V, Finnemore R, Palmer FR, Kennedy BK, et al. Rejuvant®, a potential life-extending compound formulation with alpha-ketoglutarate and vitamins, conferred an average 8 year reduction in biological aging, after an average of 7 months of use, in the TruAge DNA methylation test. Aging . 2021;13:24485–99.
- Fransquet PD, Wrigglesworth J, Woods RL, Ernst ME, Ryan J. The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clin Epigenetics. 2019;11:62.
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai P-C, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging . 2016;8:1844–65.
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25.
- Miller RA, Harrison DE, Astle CM, Bogue MA, Brind J, Fernandez E, et al. Glycine supplementation extends lifespan of male and female mice. Aging Cell. 2019;18:e12953.
- Brind J, Malloy V, Augie I, Caliendo N, Vogelman JH, Zimmerman JA, et al. Dietary glycine supplementation mimics lifespan extension by dietary methionine restriction in Fisher 344 rats. FASEB J [Internet]. Wiley; 2011;25. Available from: https://onlinelibrary.wiley.com/doi/10.1096/fasebj.25.1_supplement.528.2
- Liu YJ, Janssens GE, McIntyre RL, Molenaars M, Kamble R, Gao AW, et al. Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet. 2019;15:e1007633.
- Obata F, Miura M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat Commun. 2015;6:8332.
- Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem. 2009;284:22507–11.
- Sugiyama K, Kushima Y, Muramatsu K. Effect of dietary glycine on methionine metabolism in rats fed a high-methionine diet. J Nutr Sci Vitaminol . 1987;33:195–205.
- Salameh Y, Bejaoui Y, El Hajj N. DNA Methylation Biomarkers in Aging and Age-Related Diseases. Front Genet. 2020;11:171.
- Ruiz-Ramírez A, Ortiz-Balderas E, Cardozo-Saldaña G, Diaz-Diaz E, El-Hafidi M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin Sci . 2014;126:19–29.
- Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, et al. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011;94:847–53.
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–40.
- Gheller BJ, Blum JE, Lim EW, Handzlik MK, Hannah Fong EH, Ko AC, et al. Extracellular serine and glycine are required for mouse and human skeletal muscle stem and progenitor cell function. Mol Metab. 2021;43:101106.
- Lin C, Han G, Ning H, Song J, Ran N, Yi X, et al. Glycine Enhances Satellite Cell Proliferation, Cell Transplantation, and Oligonucleotide Efficacy in Dystrophic Muscle. Mol Ther. 2020;28:1339–58.
- Shintani H, Ashida H, Shintani T. Shifting the focus of d-glucosamine from a dietary supplement for knee osteoarthritis to a potential anti-aging drug. Human Nutrition & Metabolism. 2021;26:200134.
- Li Z-H, Gao X, Chung VC, Zhong W-F, Fu Q, Lv Y-B, et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Ann Rheum Dis. 2020;79:829–36.
- Ma H, Li X, Sun D, Zhou T, Ley SH, Gustat J, et al. Association of habitual glucosamine use with risk of cardiovascular disease: prospective study in UK Biobank. BMJ. 2019;365:l1628.
- Bell GA, Kantor ED, Lampe JW, Shen DD, White E. Use of glucosamine and chondroitin in relation to mortality. Eur J Epidemiol. 2012;27:593–603.
- Pocobelli G, Kristal AR, Patterson RE, Potter JD, Lampe JW, Kolar A, et al. Total mortality risk in relation to use of less-common dietary supplements. Am J Clin Nutr. 2010;91:1791–800.
- Weimer S, Priebs J, Kuhlow D, Groth M, Priebe S, Mansfeld J, et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun. 2014;5:3563.
- Henrotin Y, Mobasheri A, Marty M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res Ther. 2012;14:201.
- Yang W, Sun C, He SQ, Chen JY, Wang Y, Zhuo Q. The Efficacy and Safety of Disease-Modifying Osteoarthritis Drugs for Knee and Hip Osteoarthritis-a Systematic Review and Network Meta-Analysis. J Gen Intern Med. 2021;36:2085–93.
- Schumacher B, Pothof J, Vijg J, Hoeijmakers JHJ. The central role of DNA damage in the ageing process. Nature. 2021;592:695–703.
- Åkesson C, Lindgren H, Pero RW, Leanderson T, Ivars F. Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100® [Internet]. International Immunopharmacology. 2005. p. 219–29. Available from: http://dx.doi.org/10.1016/j.intimp.2004.09.028
- Pero, Lund. In vivo treatment of humans with quinic acid enhances DNA repair and reduces the influence of lifestyle factors on risk to disease. Int J Biotechnol Biochem [Internet]. Available from: https://dna-360.com/pdf/ac11_research_peroquinmax.pdf
- Sheng Y, Li L, Holmgren K, Pero RW. DNA repair enhancement of aqueous extracts of Uncaria tomentosa in a human volunteer study. Phytomedicine. 2001;8:275–82.
- Pero RW, Giampapa V, Vojdani A. Comparison of a Broad Spectrum Anti-Aging Nutritional Supplement with and without the Addition of a DNA Repair Enhancing Cat’s Claw Extract. J Anti Aging Med. Mary Ann Liebert, Inc., publishers; 2002;5:345–53.
- Sheng Y, Bryngelsson C, Pero RW. Enhanced DNA repair, immune function and reduced toxicity of C-MED-100TM, a novel aqueous extract from Uncaria tomentosa. J Ethnopharmacol. 2000;69:115–26.
- Åkesson C, Pero RW, Ivars F. C-Med 100®, a hot water extract of Uncaria tomentosa, prolongs lymphocyte survival in vivo. Phytomedicine. 2003;10:23–33.
- Guthrie OW, Gearhart CA, Fulton S, Fechter LD. Carboxy alkyl esters of Uncaria tomentosa augment recovery of sensorineural functions following noise injury. Brain Res. 2011;1407:97–106.
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, Inflammation and Immunity. Nutrients. 2016;8:167.
- Ganesan S, Faris AN, Comstock AT, Wang Q, Nanua S, Hershenson MB, et al. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94:258–71.
- Wu W, Li R, Li X, He J, Jiang S, Liu S, et al. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses [Internet]. 2015;8. Available from: http://dx.doi.org/10.3390/v8010006
- Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, Ortiz M, O’Sullivan CK, Fernández-Larrea JB. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1-6 cells to a liposome model. J Agric Food Chem. 2014;62:8085–93.
- US Burden of Disease Collaborators, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, et al. The State of US Health, 1990-2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA. 2018;319:1444–72.
- Ahmad FB, Anderson RN. The Leading Causes of Death in the US for 2020. JAMA. 2021;325:1829–30.
- Bouamama S, Bouamama A. Quercetin handles cellular oxidant / antioxidant systems and mitigates immunosenescence hallmarks in human PBMCs: an in vitro study [Internet]. bioRxiv. 2022 [cited 2022 Jul 15]. p. 2021.10.23.465570. Available from: https://www.biorxiv.org/content/10.1101/2021.10.23.465570v2
- Heinz SA, Henson DA, Austin MD, Jin F, Nieman DC. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol Res. 2010;62:237–42.
- Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur J Drug Metab Pharmacokinet. 2019;44:169–77.
- Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28.
- Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal. 2013;19:151–62.
- Wang L, Cao D, Wu H, Jia H, Yang C, Zhang L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin Appl Thromb Hemost. 2019;25:1076029619871359.
- Farsad-Naeimi A, Alizadeh M, Esfahani A, Darvish Aminabad E. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018;9:2025–31.
- Li Y-R, Li S, Lin C-C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 2018;44:69–82.
- McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:575482.
- Hougee S, Faber J, Sanders A, de Jong RB, van den Berg WB, Garssen J, et al. Selective COX-2 inhibition by a Pterocarpus marsupium extract characterized by pterostilbene, and its activity in healthy human volunteers. Planta Med. 2005;71:387–92.
- Obrador E, Salvador-Palmer R, Jihad-Jebbar A, López-Blanch R, Dellinger TH, Dellinger RW, et al. Pterostilbene in Cancer Therapy. Antioxidants (Basel) [Internet]. 2021;10. Available from: http://dx.doi.org/10.3390/antiox10030492
- Lin W-S, Leland JV, Ho C-T, Pan M-H. Occurrence, Bioavailability, Anti-inflammatory, and Anticancer Effects of Pterostilbene. J Agric Food Chem. 2020;68:12788–99.
- Ma Z, Zhang X, Xu L, Liu D, Di S, Li W, et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol Res. 2019;145:104265.
- Poulose SM, Thangthaeng N, Miller MG, Shukitt-Hale B. Effects of pterostilbene and resveratrol on brain and behavior. Neurochem Int. 2015;89:227–33.
- Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, et al. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66.
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201.
- Stahl W. Macular carotenoids: lutein and zeaxanthin. Dev Ophthalmol. 2005;38:70–88.
- Widomska J, Subczynski WK. Mechanisms enhancing the protective functions of macular xanthophylls in the retina during oxidative stress. Exp Eye Res. 2019;178:238–46.
- Liu R, Wang T, Zhang B, Qin L, Wu C, Li Q, et al. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;56:252–8.
- Wang X, Jiang C, Zhang Y, Gong Y, Chen X, Zhang M. Role of lutein supplementation in the management of age-related macular degeneration: meta-analysis of randomized controlled trials. Ophthalmic Res. 2014;52:198–205.
- Huang Y-M, Dou H-L, Huang F-F, Xu X-R, Zou Z-Y, Lin X-M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed Res Int. 2015;2015:564738.
- Huang Y-M, Dou H-L, Huang F-F, Xu X-R, Zou Z-Y, Lu X-R, et al. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: a randomised, double-blind, placebo-controlled trial. Br J Ophthalmol. 2015;99:371–5.
- Liu X-H, Yu R-B, Liu R, Hao Z-X, Han C-C, Zhu Z-H, et al. Association between lutein and zeaxanthin status and the risk of cataract: a meta-analysis. Nutrients. 2014;6:452–65.
- Ma L, Hao Z-X, Liu R-R, Yu R-B, Shi Q, Pan J-P. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch Clin Exp Ophthalmol. 2014;252:63–70.
- Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8:156–62.
- Arnal E, Miranda M, Barcia J, Bosch-Morell F, Romero FJ. Lutein and docosahexaenoic acid prevent cortex lipid peroxidation in streptozotocin-induced diabetic rat cerebral cortex. Neuroscience. 2010;166:271–8.
- Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, et al. Relationship between Serum and Brain Carotenoids, α-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J Aging Res. 2013;2013:951786.
- Nouchi R, Suiko T, Kimura E, Takenaka H, Murakoshi M, Uchiyama A, et al. Effects of Lutein and Astaxanthin Intake on the Improvement of Cognitive Functions among Healthy Adults: A Systematic Review of Randomized Controlled Trials. Nutrients [Internet]. 2020;12. Available from: http://dx.doi.org/10.3390/nu12030617
- Stringham NT, Holmes PV, Stringham JM. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol Behav. 2019;211:112650.
- Widomska J, Subczynski WK. Why has Nature Chosen Lutein and Zeaxanthin to Protect the Retina? J Clin Exp Ophthalmol. 2014;5:326.
- Wisniewska A, Subczynski WK. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic Biol Med. 2006;40:1820–6.
- Subczynski WK, Wisniewska A, Widomska J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch Biochem Biophys. 2010;504:61–6.
- Subczynski WK, Wisniewska-Becker A, Widomska J. Can macular xanthophylls replace cholesterol in formation of the liquid-ordered phase in lipid-bilayer membranes? Acta Biochim Pol. 2012;59:109–14.
- Subczynski WK, Markowska E, Sielewiesiuk J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim Biophys Acta. 1991;1068:68–72.
- Subczynski WK, Widomska J, Feix JB. Physical properties of lipid bilayers from EPR spin labeling and their influence on chemical reactions in a membrane environment. Free Radic Biol Med. 2009;46:707–18.
- Siems WG, Sommerburg O, van Kuijk FJ. Lycopene and beta-carotene decompose more rapidly than lutein and zeaxanthin upon exposure to various pro-oxidants in vitro. Biofactors. 1999;10:105–13.
- Socaciu C, Jessel R, Diehl HA. Carotenoid incorporation into microsomes: yields, stability and membrane dynamics. Spectrochim Acta A Mol Biomol Spectrosc. 2000;56:2799–809.
- Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62.
- Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science [Internet]. 2018;359. Available from: http://dx.doi.org/10.1126/science.aan2788
- Madeo F, Carmona-Gutierrez D, Kepp O, Kroemer G. Spermidine delays aging in humans. Aging . 2018;10:2209–11.
- Madeo F, Bauer MA, Carmona-Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2019;15:165–8.
- Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108:371–80.
- Schroeder S, Hofer SJ, Zimmermann A, Pechlaner R, Dammbrueck C, Pendl T, et al. Dietary spermidine improves cognitive function. Cell Rep. 2021;35:108985.
- Schwarz C, Horn N, Benson G, Wrachtrup Calzado I, Wurdack K, Pechlaner R, et al. Spermidine intake is associated with cortical thickness and hippocampal volume in older adults. Neuroimage. 2020;221:117132.
- Wirth M, Benson G, Schwarz C, Köbe T, Grittner U, Schmitz D, et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex. 2018;109:181–8.
- Freitag K, Sterczyk N, Wendlinger S, Obermayer B, Schulz J, Farztdinov V, et al. Spermidine reduces neuroinflammation and soluble amyloid beta in an Alzheimer’s disease mouse model. J Neuroinflammation. 2022;19:172.
- Xu T-T, Li H, Dai Z, Lau GK, Li B-Y, Zhu W-L, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging . 2020;12:6401–14.
- Cheah IK, Halliwell B. Ergothioneine, recent developments. Redox Biol. 2021;42:101868.
- Kumosani TA. L-ergothioneine level in red blood cells of healthy human males in the Western province of Saudi Arabia. Exp Mol Med. 2001;33:20–2.
- Kawano H, Otani M, Takeyama K, Kawai Y, Mayumi T, Hama T. Studies on ergothioneine. VI. Distribution and fluctuations of ergothioneine in rats. Chem Pharm Bull . 1982;30:1760–5.
- Cheah IK, Feng L, Tang RMY, Lim KHC, Halliwell B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem Biophys Res Commun. 2016;478:162–7.
- Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103:17589–94.
- Ames BN. Optimal micronutrients delay mitochondrial decay and age-associated diseases. Mech Ageing Dev. 2010;131:473–9.
- Smith E, Ottosson F, Hellstrand S, Ericson U, Orho-Melander M, Fernandez C, et al. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart. 2020;106:691–7.
- Ames BN. Prolonging healthy aging: Longevity vitamins and proteins. Proc Natl Acad Sci U S A. 2018;115:10836–44.
- Beelman RB, Kalaras MD, Phillips AT, Richie JP Jr. Is ergothioneine a “longevity vitamin” limited in the American diet? J Nutr Sci. 2020;9:e52.
- Zarse K, Terao T, Tian J, Iwata N, Ishii N, Ristow M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur J Nutr. 2011;50:387–9.
- Fajardo VA, LeBlanc PJ, Fajardo VA. Trace lithium in Texas tap water is negatively associated with all-cause mortality and premature death. Appl Physiol Nutr Metab. 2018;43:412–4.
- Coutts F, Palmos AB, Duarte RRR, de Jong S, Lewis CM, Dima D, et al. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacology. 2019;44:757–65.
- Nespital T, Neuhaus B, Mesaros A, Pahl A, Partridge L. Lithium can mildly increase health during ageing but not lifespan in mice. Aging Cell. 2021;20:e13479.
- Salarda EM, Zhao NO, Lima CNNC, Fries GR. Mini-review: The anti-aging effects of lithium in bipolar disorder. Neurosci Lett. 2021;759:136051.
- Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198:351–6.



